
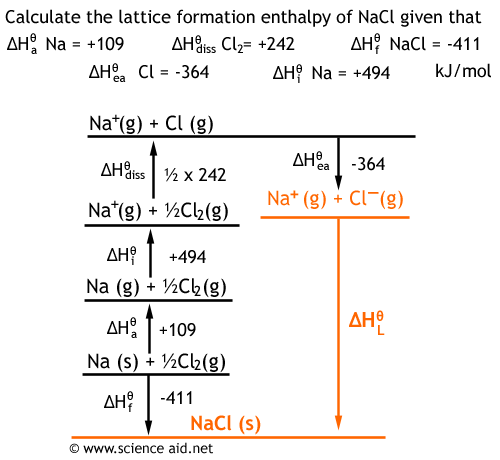
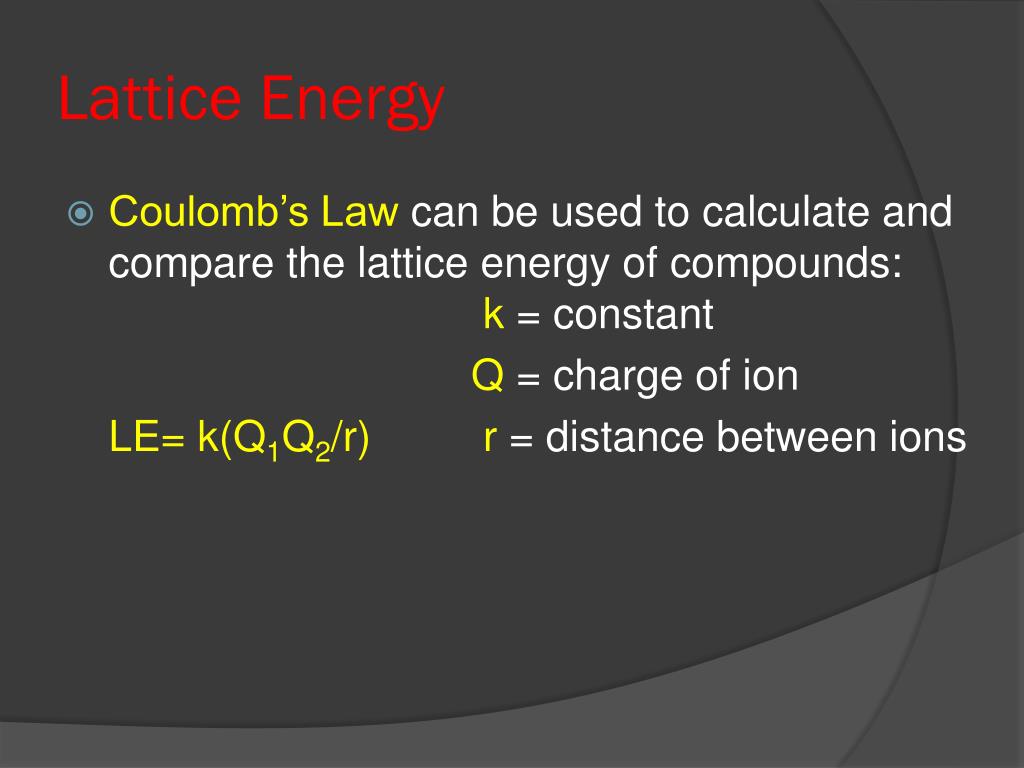
In both of the compounds, Na+ ion is commmon. And we should get negative 2859 kilojoules. Lattice enthalpy of ionic compound is inversely proportional to sum of the ionic radius of cation and anion. To calculate lattice energy from the Born-Haber cycle, we use the equation below: Lattice Energy (U) H f - ( H s u b + H d i s s + I E + E A) So, if we were to use the different energy values to calculate the energy, we would get -788 kJ. We can solve this equation for the lattice energy of lithium oxide. Born-haber cycle of NaCl, Isadora Santos - StudySmarter Originals. According to this equation, stronger interactions occur between ions with larger charges and smaller radii. The two main factors that affect the lattice energy of ionic compounds are the magnitude of charge and the distance between the ions. As an example, the lattice energy of sodium chloride, NaCl, is the energy released when gaseous Na+ and Cl ions come together to form a lattice of alternating ions in the NaCl crystal. Lattice energy depends on the strength of interactions between cations and anions in the lattice, which we can estimate using Coulomb's law: F (qq)/r. defines the lattice enthalpy of NaCl.Thus by using the Born- Haber cycle, one can determine the lattice enthalpy of an ionic compound. Note: Lattice energy is the energy which is released when two oppositely charged ions attract each other to form an ionic solid. In order to determine the lattice energy associated with the crystal AB, substitute the charge of A and B in the equation and then reorder the equation in terms of the lattice energy of NaCl. The radius of the ions The lattice enthalpy of magnesium oxide is also increased relative to sodium chloride because magnesium ions are smaller than sodium. It is directly proportional to the product of the ion charges and inversely proportional to the internuclear distance. Hint: The energy associated with the crystal lattice of a compound is called the lattice energy. Lattice energy depends on the strength of interactions between cations and anions in the lattice, which we can estimate using Coulomb's law: F.


 0 kommentar(er)
0 kommentar(er)
